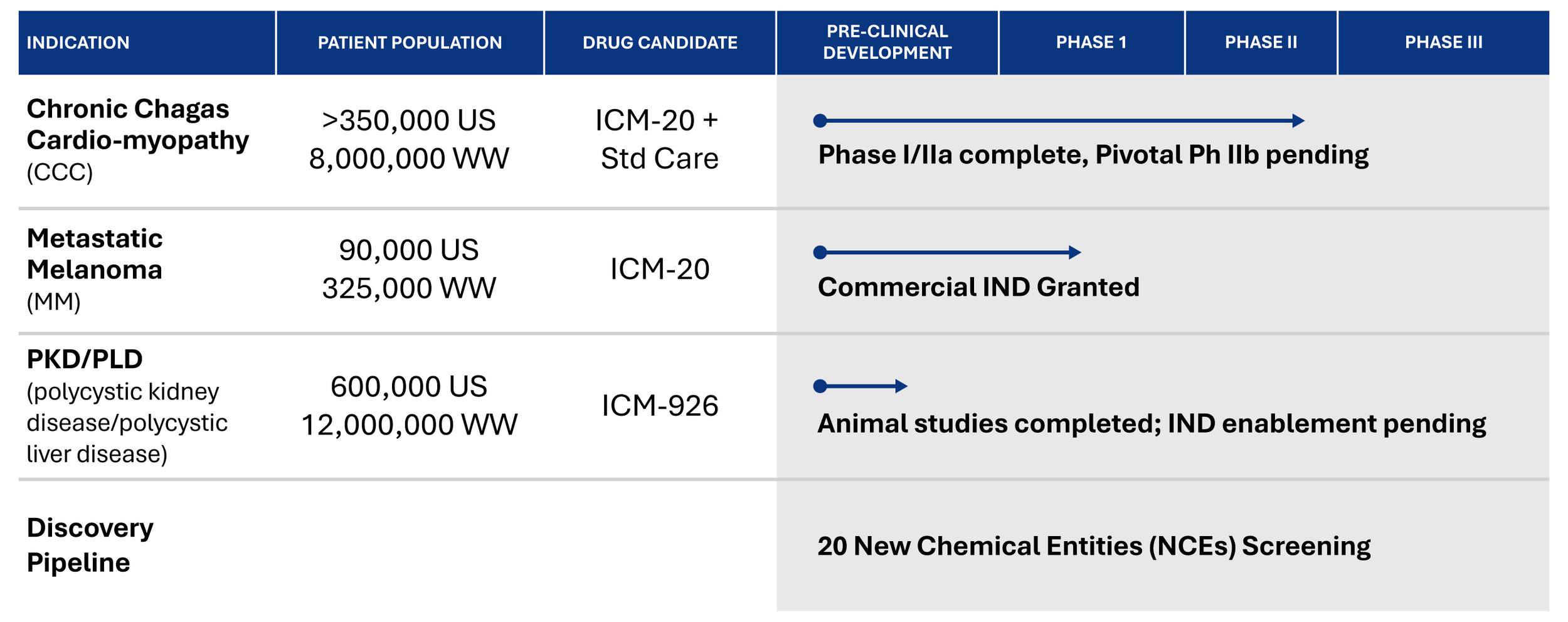
Product Pipeline
w/Opportunities in Oncology, Kidney and Liver Diseases
Why Begin with Chronic Chagas Cardiomyopathy?
Caused by infection from parasites with a single targetable pathogenic mitochondrion
Approximately 350,000 patients in the US; 8M worldwide
Very large unmet medical need, demonstrating our mechanism
Potential FDA priority indication could provide regulatory advantage
ICM-20 is clinically validated w/proven safety & biomarker efficacy
GMP formulation to FDA guidelines –
Low cost, scalable, exclusive US production capacity
Clinical Progress: Translating Discovery into Breakthrough Treatments At ICM Therapeutics, we are not just theorizing—we are proving it in the clinic.
• Phase I/IIa clinical trials have established the safety of our scientific rationale
• A clinically practical approach backed by decades of research and collaborations with leading scientific institutions.
• We are advancing to Phase IIb studies targeting infectious cardiomyopathy where single, hyperpolarized mitochondria cause disease.
Based on a scalable drug platform and deep expertise in mitochondrial medicine, ICM is pioneering a new frontier in disease treatment, with expansion opportunities in cancer and immune-driven diseases.